Spinal Cord Stimulator (Dorsal Column Stimulator)
This treatment involves using electrical stimulation against the spinal cord to minimize transmission of pain signals from the body to the brain.
Quite often, patients report that they will feel a slight tingling, but the pain is significantly better.
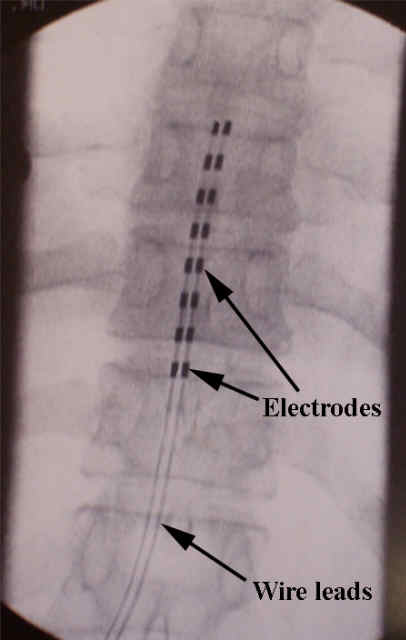
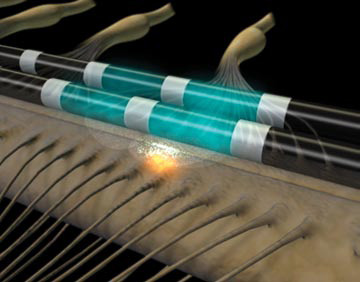
The procedure is done in two stages.
First, there is a trial where wires are threaded up within the spinal canal (the space inside the spinal bones where the nerves reside) but outside of the dura (the layer that keeps the spinal fluid around the spinal cord).
The trial period lasts 3-7 days, and the patient can assess whether the stimulator will help relieve the pain.
After the trial period, the trial leads are removed.
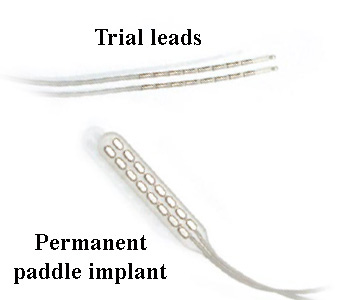
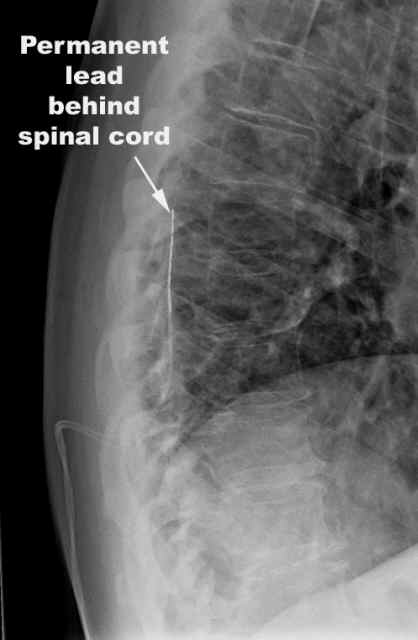
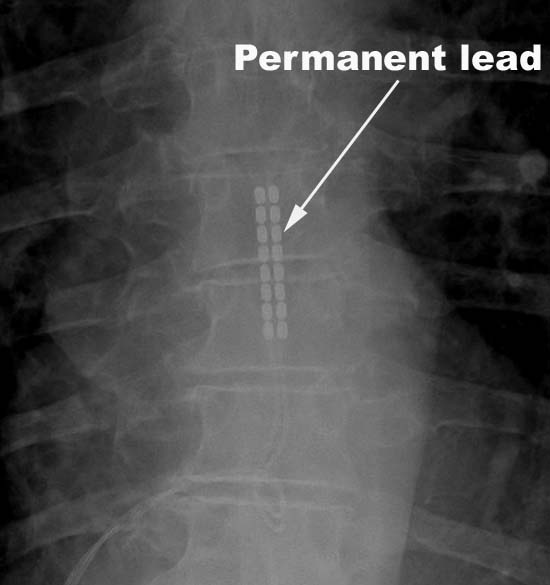
If the trial is successful, a paddle lead is usually implanted. This paddle has the electrodes embedded in the surface, and is less likely to move around.
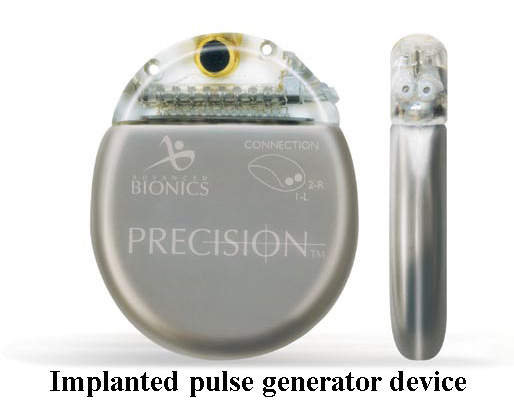
The power supply for the leads during the trial period is worn outside the body, but if the trial is successful and a permanent implant is chosen, the implantable pulse generator (IPG) is buried in the soft tissue and can be recharged and controlled through the skin. The device that is implanted is a little larger than an Oreo cookie.
Frequently Asked Questions:
What is this spinal cord stimulator and how does it work?
The stimulator is a specialized device which applies a low level of current to the back part of the spinal cord (dorsal columns) by some wires or electrodes.
very close to the spinal cord. The device works by interrrupting nerve impulses (many of which convey pain sensations) on their way to the brain.
Which patients are appropriate for these stimulators?
This treatment is best used for patients with chronic and severe pain who have not responded to other treatment options. Conditions that can be treated include continued back and leg pain after back surgeries, or other pain syndromes (reflex sympathetic dystrophy, complex regional pain syndrome).
HOWEVER, recent moves by insurers, like here, indicate insurers are looking to not cover this treatment, despite its success.
Frequently Asked Questions:
What is involved in doing a trial?
If you have heard of the type of anesthesia pregnant women get called an epidural, the anatomical
considerations are very similar. For the pregnant ladies, they get a catheter (tube) in the epidural space to deliver medicine. For spinal stimulator trials, one or two wires are delivered into the same space. The wires are not permanent. The patient usually knows within a few hours if the stimulation will make them feel better. The wires are usually placed with a local anesthetic with xray (fluoroscopic) guidance.
How is the permanent implant put in place?
Usually this implant is done in the operating room under general anesthesia, but can also be done with local anesthesia and sedation.
Who should not have this procedure?
Patients who have a history of spinal infection should not have this procedure. Patients who are dependent on coumadin or Plavix will have a higher risk of bleeding in their spinal canal and probably this procedure should be avoided, but these cases are considered on a case by case basis.
Frequently Asked Questions:
What should I expect after the procedure?
After either the trial or the permanent lead placement, there is usually some soreness either from the needles or the surgery to place the pulse generator, but usually the pre-procedure pain is better. Most patients report that they can feel the stimulator working. Typically, patients report a 50-70% reduction in their pain, and can use less narcotic medication.
How long do the batteries last?
With newer technology, batteries last many years, often more than ten, and are rechargeable from outside the body.
What are the risks of this procedure?
While as spine procedures go, placement of a spinal stimulator is relatively mild, but the risks in general are outlined here. Probably the biggest risk with this procedure is infection, which could occur at at later time.
